Wilhelm Roentgen
A new form of radiation was discovered in 1895 by Wilhelm Roentgen, a German physicist. He called it X-radiation to denote its unknown nature. This mysterious radiation had the ability to pass through many materials that absorb visible light. X-rays also have the ability to knock electrons loose from atoms. Over the years these exceptional properties have made X-rays useful in many fields, such as medicine and research into the nature of the atom.
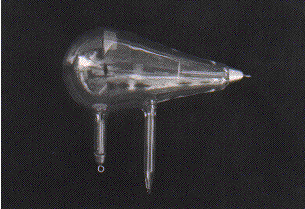
Roentgen was working in his laboratory at the Physical Institute of the University of Würzburg, Germany, experimenting with a Crookes tube.
This tube is a glass bulb with positive and negative electrodes, evacuated of air, which displays a fluorescent glow when a high voltage current is passed though it. When he shielded the tube with heavy black cardboard, he found that a greenish fluorescent light could be seen from a platinobaium screen 9 feet away.
This tube is a glass bulb with positive and negative electrodes, evacuated of air, which displays a fluorescent glow when a high voltage current is passed though it. When he shielded the tube with heavy black cardboard, he found that a greenish fluorescent light could be seen from a platinobaium screen 9 feet away.
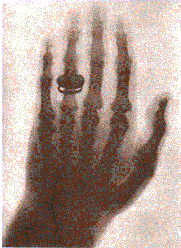
He concluded that a new type of ray emitted from the tube, passed through the covering, and casted shadows of solid objects. The rays passes through most substances, including the soft tissues of the body, but left the bones and most metals visible. One of his earliest photographic plate from his experiments was a film of his wife, Bertha’s hand with a ring, was produced on Friday, November 8, 1895.
On Saturday, December 28, 1895 Roentgen submitted his first “provisorial” communication, Ueber eine nue Art von Strahlen (On a New Kind of Rays) in the Proceedings of the Würzburg Phisico-Medical Society. On Thursday, January 23, 1896 he made his first public presentation before the same society. After the lecture Roentgen made a plate of the hand a famous anatomist named Kölliker, who proposed that the new discovery be named Roentgen’s Rays.
The news spread rapidly through out the world. As early as February 8, 1896, X-rays were being used clinically the United States. in Dartmouth, Massachusetts when Edwin Brant Frost produced a plate of a Colles fracture in a man named Eddie McCarthy for his brother, Dr. Gilman Dubois Frost.
Eventually, X-rays were found to be another form of light. Light is the by-product of the constant jiggling, vibrating, hurly-burly of all matter.
Like a frisky puppy, matter cannot be still. The chair you are sitting in may look and feel motionless. But if you could see down to the atomic level you would see atoms and molecules vibrating hundreds of trillions of times a second and bumping into each other, while electrons zip around at speeds of 25,000 miles per hour.
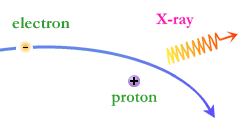
When charged particles collide–or undergo sudden changes in their motion–they produce bundles of energy called photons that fly away from the scene of the accident at the speed of light. In fact they are light, or electromagnetic radiation, to use the technical term. Since electrons are the lightest known charged particle, they are most fidgety, so they are responsible for most of the photons produced in the universe.
The energy of the photon tells what kind of light it is. Radio waves are composed of low energy photons. Optical photons–the only photons perceived by the human eye–are a million times more energetic than the typical radio photon. The energies of X-ray photons range from hundreds to thousands of times higher than that of optical photons.
The speed of the particles when they collide or vibrate sets a limit on the energy of the photon. The speed is also a measure of temperature. (On a hot day, the particles in the air are moving faster than on a cold day.)
Very low temperatures (hundreds of degrees below zero Celsius) produce low energy radio and microwave photons, whereas cool bodies like ours (about 30 degrees Celsius) produce infrared radiation. Very high temperatures (millions of degrees Celsius) produce X-rays.
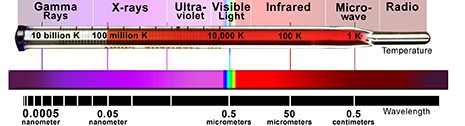
The Electromagnetic Spectrum. The wavelength of radiation produced by an object is usually related to its temperature.
The photons themselves can also collide with electrons. If the electrons have more energy than the photons, the collision can boost the energy of the photons. In this way, photons can be changed from low-energy photons to high-energy photons. This process, called Compton scattering, is thought to be important around black holes, where matter is dense and has been heated to many millions of degrees.
The photons collected in space by X-ray telescopes reveal the hot spots in the universe–regions where particles have been energized or raised to high temperatures by gigantic explosions or intense gravitational fields.
Synchrotron Radiation
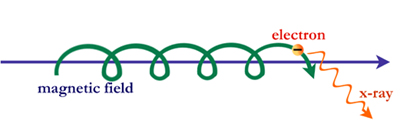
But this is not the whole story. X-ray photons can also be created under different conditions. When physicists were operating the first particle accelerators, they discovered that electrons can produce photons without colliding at all. This was possible because the magnetic field in the accelerators was causing the electrons to move in large spirals around magnetic field lines of force. This process is called synchrotron radiation.
In the cosmos particles such as electrons can be accelerated to high energies– near the speed of light– by electric and magnetic fields. These high-energy particles can produce synchrotron photons with wavelengths ranging from radio up through x-ray and gamma-ray energies.
Synchrotron radiation from cosmic sources has a distinctive spectrum, or distribution of photons with energy. The radiation falls off with energy less rapidly than does the spectrum of radiation from a hot gas. When synchrotron radiation is observed in supernova remnants, cosmic jets, or other sources, it reveals information about the high-energy electrons and magnetic fields that are present.