The World of Atomic Clocks
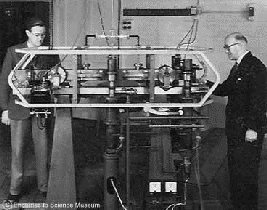
The 1955 Cesium Atomic Clock at the National Physical Laboratory, UK. It kept time to a second in 300 years.
A “cesium(-beam) atomic clock” (or “cesium-beam frequency standard”) is a device that uses as a reference the exact frequency of the micro- wave spectral line emitted by atoms of the metallic element cesium, in particular its isotope of atomic weight 133 (“Cs-133”). The integral of frequency is time, so this frequency,9,192,631,770 hertz (Hz = cycles/second), provides the fundamental unit of time, which may thus be measured by cesium clocks.
Today, cesium clocks measure frequency with an accuracy of from 2 to 3 parts in 10 to the 14th, i.e. 0.0002 Hz; this corresponds to a time measurement accuracy of 2 nanoseconds per day or one second in 1,400,000 years. It is the most accurate realization of a unit that mankind has yet achieved. A cesium clock operates by exposing cesium atoms to microwaves until they vibrate at one of their resonant frequencies and then counting the corresponding cycles as a measure of time. The frequency involved is that of the energy absorbed from the incident photons when they excite the outermost electron in a cesium atom to jump (“transition”) from a lower to a higher orbit.
According to quantum theory, atoms can only exist in certain discrete (“quantized”) energy states depending on what orbits about their nuclei are occupied by their electrons. Different transitions are possible; those in question refer to a change in the electron and nuclear spin (“hyperfine”) energy level of the lowest set of orbits called the “ground state.” Cesium is the best choice of atom for such a measurement because all of its 55 electrons but the outermost are confined to orbits in stable shells of electromagnetic force. Thus, the outermost electron is not disturbed much by the others. The cesium atoms are kept in a very good vacuum of about 10 trillionths of an atmosphere so that the cesium atoms are little affected by other particles. All this means that they radiate in a narrow spectral line whose wavelength or frequency can be accurately determined.
In a cesium clock , liquid cesium is heated to a gaseous state in an oven. A hole in the oven allows the atoms to escape at high speed. These particles pass between two electromagnets whose field causes the atoms to separate into two beams, depending on which spin energy state they are in. Those in the lower energy state pass through the ends of a U-shaped cavity in which they are irradiated by microwaves of 3.26-cm wavelength.
The absorption of these micro- waves excite transitions of many of the atoms from the lower to the higher energy state. The beam continues through another pair of electromagnets, whose field again divides up the beam. Those atoms in the higher energy state strike a hot wire, which ionizes them. Thereafter, a mass spectrometer selects only the cesium atoms from any impurities and directs them onto an electron multiplier.
The frequency of the microwaves is adjusted until the electron multiplier output current is maximized, constituting the measurement of the atoms’ resonance frequency . This frequency is elctronically divided down and used in a feedback control circuit (“servo-loop”) to keep a quartz crystal oscillator locked to a frequency of 5 megahertz (MHz), which is the actual output of the clock, along with a one-pulse-per-second signal. The entire apparatus is shielded from external magnetic fields.
The first method for accurately measuring hyperfine frequencies by molecular beam resonance was developed by I.I. Rabi and his associates in 1937 at Columbia University. The first molecular clock, using ammonia gas, was built by H. Lyons at the National Bureau of Standards in 1949. The first atomic clock, a cesium-beam frequency standard, was built starting in 1949 and was first operated in 1951, resulting in the first direct measurements of cesium hyperfine frequencies. The clock, called NBS-1, was the first in a series that is now up to 7 (NBS is now the National Institute of Standards and Technology, so their latest standard is called NIST-7). Between 1953 and 1955, L. Essen and J.V.L. Parry of the National Physical Laboratory (NPL) in Teddington, England built cesium atomic clock. These atomic clocks were later refined by others, notably N.F. Ramsey and J.R. Zacharias, and are the primary standards referred to above.
The first atomic clock timescale was established in 1955 at NPL, though it differed signicantly from the astronomical ephemeris timescale established by Dr. William Markowitz, head of Time Service at the U.S. Naval Observatory. Markowitz and Essen collaborated on the determination of a best value for the cesium hyperfine frequency, and in 1958 they reported a value of 9,192,631,770 hertz (cycles/second).
The Second
In 1967, the 13th General Conference on Weights and Measures first defined the International System (SI) unit of time, the second, in terms of atomic time rather than the motion of the Earth. Specifically, a second was defined as the duration of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of cesium-133 atoms in their ground state undisturbed by external fields .
The first commercial atomic frequency standards were built by R. Daly of National Company and J. Holloway of Varian Associates. Their cesium tube design was incorporated by L. Cutler and his coworkers at Hewlett-Packard in what would become the largest selling series of commercial standards.
Recent improvements in cesium clock technology include replacement of the state-selection magnets with laser beams, which can select and detect the required transition with greater efficiency and less motion of, and hence less noise from, the radiating atoms. A new appraoch is being explored called the Cesium Fountain.
Cesium Atomic Fountain Frequency Standards
The atomic fountain works by first cooling cesium atoms to microKelvin temperatures by means of an “optical molasses” generated from 6 laser beams (two counter-propagating beams in each of the three cardinal directions), tuned to a frequency slightly below the 852 nanometer optical resonance. Atoms are then launched upwards by altering the frequency of the lasers. The launched atoms travel vertically from their trapping region, are prepared into the |3,0> state, and then they continue through a microwave cavity containing a field tuned to the 9192 MHz hyperfine frequency of the cesium atom (set by the energy difference between the |4,0> and |3,0> states). They continue moving upwards until the Earth’s gravity causes them to fall back through the microwave cavity, where they again interact with the microwave field. The two exposures to the microwave field will cause some atoms to make the transition from the |3,0> state to the |4,0> state. The atoms then pass through two state detection regions where the number of atoms in the two states are measured by detecting scattered laser light.
The true frequency of the 9192 MHz microwave field can be inferred from the percentage of atoms that made the transition from the |3,0> state to |4,0> state. The most important advantage in precision (stability on the short-term, seconds to hours) of such a clock over conventional cesium frequency standards is that more precise frequency measurements can be made over the long (1/2-second) travel time between the two exposures to the microwave field. One important advantage in accuracy (stability with respect to all sources of error, not just short-term measurement-noise) comes from the fact that the interaction with the microwave field occurs in the same cavity each time, resulting in the cancellation of certain systematic effects. Another advantage is that the velocity of the atoms is so slow that the second-order Doppler shift (time dilation) is negligible.
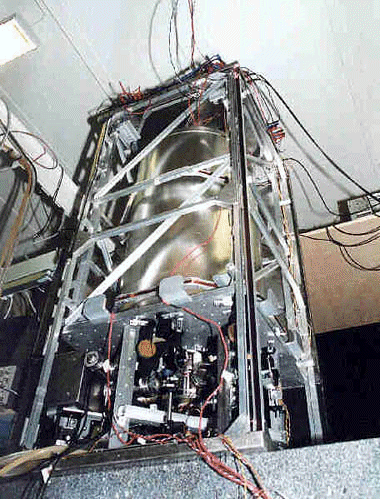
The atomic cesium fountain (1.2m high) is the first frequency standard using cold cesium atoms. Cesium atoms are cooled to a few micro Kelvin and they are launched one meter high. They follow a parabolic flight, passing twice through a single microwave cavity. The long time between the two interactions leads to a sub-Hertz Ramsey resonance, 100 times narrower than the Ramsey fringes obtained in conventional cesium frequency standards.
How Trapped Ions Can Be Used to Make a Clock
Ions can be used to set the frequency of a microwave signal, and frequencies can be converted to time by simple addition. To see how this works, you must realize that if you have a periodic signal of known frequency “f”, all you need to do is find a way to count anything that occurs once per period, and one second will be of “f” of them. This is why the most precise clocks are more accurately referred to as frequency standards. For example, if you had a voltage that went up and down past zero once each period, you could measure a second by counting the passage of “f” maxima, minima, upward-going zero crossings, or downward-going zero crossings. A specific example could be a pendulum clock. If you know that it goes back and forth every 4 seconds, then all you have to do is count the number of times it goes all the way to the right and multiply by 4 to get the number of seconds that have gone by. Of course, in practice such counting is not always so simple. Also, if the frequency is large it is usually necessary to subtract the frequency of interest from a second frequency, which yields a measurement of only the difference between two frequencies.
Ions (ionized atoms) can absorb and re-emit radiation at certain very specific frequencies. By trapping the ions, they can interact for several seconds with microwaves whose frequency can be varied so as to be “exactly” equal to, or offset by a known amount from, that frequency which the ions can absorb and re-emit. The longer the ions can interact with the microwave frequency without being disturbed, the more precisely they can distinguish between microwaves at the resonant frequency which they most strongly absorb and those at slightly different frequencies. In the case of mercury, this resonant microwave frequency is 40,507.348 MHz, which is subtracted from a similar frequency generated from the Master Clock. As described above, this frequency difference can be summed to convert into a time difference between the mercury clock and the Master Clock.
The Technique of Trapping Ions
It is well known that ions can be trapped in an oscillating quadrupole field, which is dramatized in the graphic above. As the ions move back and forth in response to the changing electrical force, it can be shown that they experience a net inward force, called the pondermotive force. Because about one million ions are trapped, this attractive force is balanced by the mutual repulsion of like charges, so that the resulting density of mercury ions is largely confined to and approximately uniform within a radius of 2.5 millimeters.
By using such a force field to trap mercury ions and the technique known as optical pumping to prepare the state of the mercury ions, it is possible to tune an input 40.5 GHz microwave field so that its frequency matches the natural frequency of the trapped mercury. Measurements of this tuned frequency forms the basis of a clock. It can be shown that the intrinsic accuracy of such a clock is chiefly affected by the second-order Doppler effect of the ionic motions (also known as time dilation).
HYDROGEN MASER CLOCKS
Masers are atomic clocks which have outstanding short-term stability. USNO’s current Sigma Tau hydrogen masers [STSC Model 2010(1994)] exhibit a time domain maximum instablility of 3.0 parts in 10 the 15th for periods of 1000 to 10,000 seconds and 2.0 parts in 10 to the 13th * t**-(3/5) for 1 sec < t < 1000 sec.
The drift is specified at initially less than 1 part in 10 to the 15th per day. The USNO Sigma Tau masers NAV-2,3,4, and 5 have shown drifts of less than a part in 10 to the 16th/day over 500 days.
These hydrogen masers are 46 x 76 x 109 cm in size and with batteries weigh 227 kg.
The main components of a hydrogen maser are the hydrogen gas supply, a controllable “leak” for the gas into the high vacuum system , a gas dischargeto produce atomic hydrogen, and a state selector which rejects atoms in the lower energy states and focusses the higher states into the storage bulb.
A small storage bottle supplies molecular hydrogen to the gas discharge bulb. In the discharge bulb, molecules of hydrogen are dissociated into atomic hydrogen. They then pass through a source collimator and a magnetic state selector which filters atoms at the desired atomic state and passes them to the resonance cavity.
The storage bulb is a quartz bulb about 20 cm tall which is coated inside with Teflon to control the recombination rate of atoms into molecules. The quartz bulb is located in a precisely machined pure copper cylinder which acts as a microwave resonant cavity for the 1.420 GHz frequency of the 21 cm hydrogen line. Once the atoms enter the resonance cavity, they find other atoms radiating and they fall in step. They “start to talk to each other” and echo what they hear. This produces a highly coherent oscillation. This is the signal to which the crystal oscillator is phase-locked. All of that is packaged in cabinets with power supplies, temperature control, and magnetic shielding.
Great care is required to keep environmental disturbances small so that the full performance potential of these sophisticated clocks can be realized.